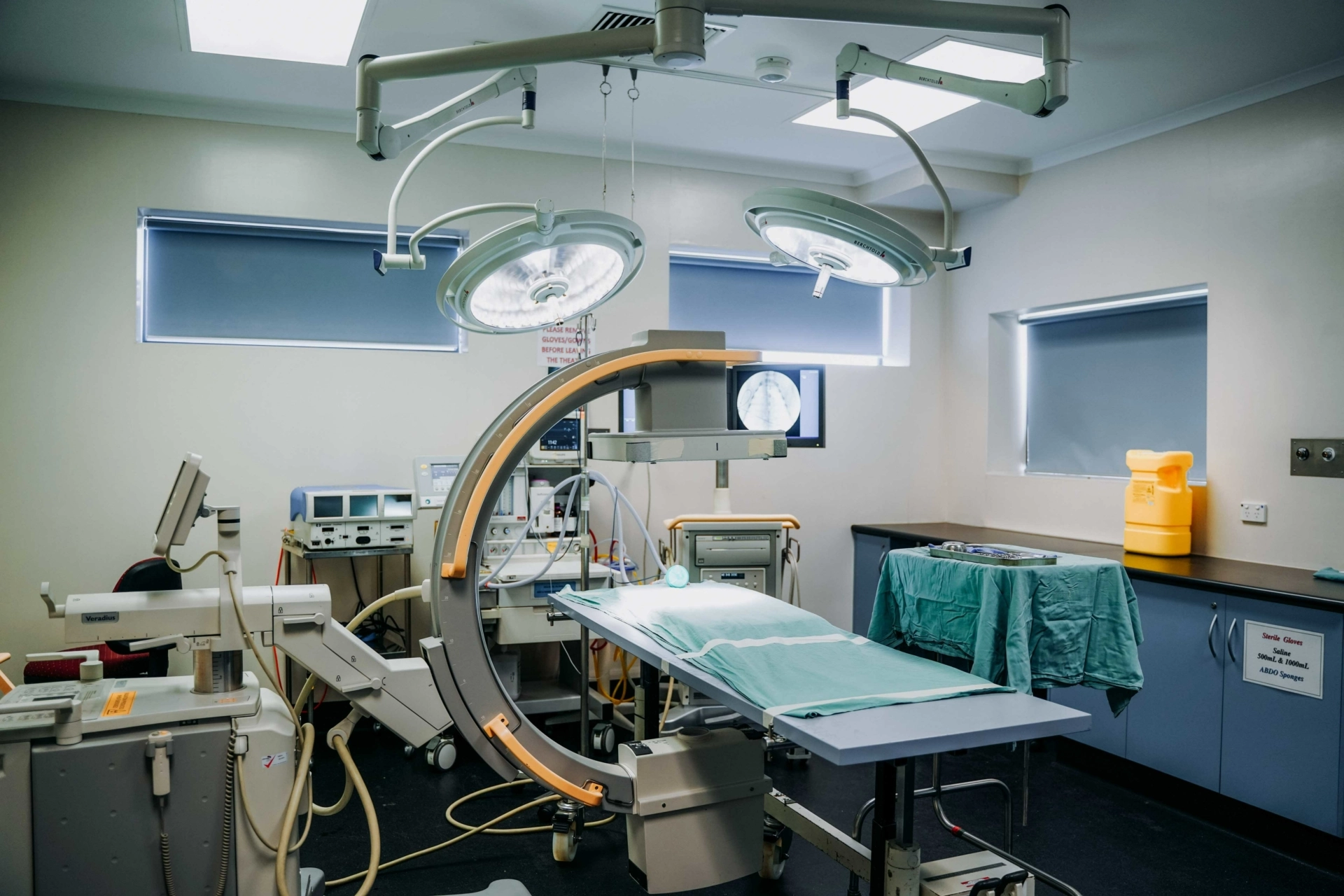
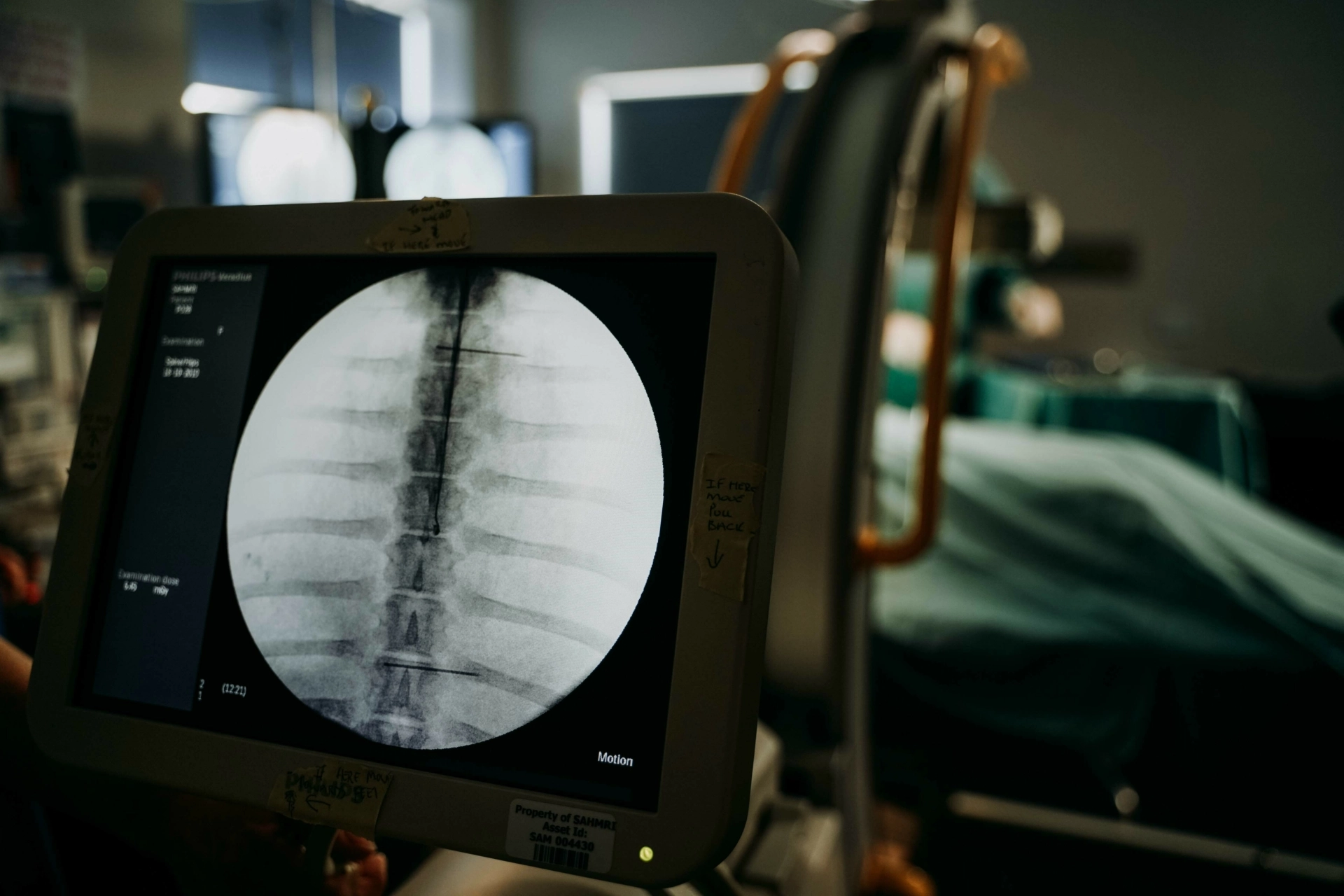
SAHMRI Preclinical, Imaging and Research Laboratories (PIRL) has played a key role in the validation of a revolutionary new medical device helping clinicians place pacemaker leads more accurately and efficiently, making the procedure safer for patients.
Fully designed, developed, and manufactured in South Australia, TorqView was created by local startup, Cardiovasc.Tech, under the leadership of biomedical engineer, Darius Chapman.
The device was recently deployed for the first time to complete clinical procedures at Flinders Private Hospital and Flinders Medical Centre, marking a leap forward in cardiac care and medical technology.
TorqView’s real-time feedback system aims to improve clinical outcomes by enhancing the precision of pacemaker lead deployment, reducing risks, and contributing to advancements in cardiac research and practice.
A NATA-recognised GLP facility with a strong track record in medical research, PIRL conducted vital preclinical safety and efficacy research for TorqView, providing the rigorous testing environment necessary to ensure the device met the highest scientific and regulatory standards before reaching clinical application.
PIRL Director, Dr Adam O’Connell, says the achievement is a great example of the type of innovation the local medtech industry is capable of producing.
“TorqView is a fantastic achievement by Cardiovasc.Tech that demonstrates the efficiency of our state’s collaborative pipeline, from preclinical research to end-user application,” Dr O’Connell said.
“South Australia’s medical research and manufacturing capabilities are world-class and contributing to that capacity is something we’re very proud of at PIRL.”
PIRL’s facilities offer comprehensive support for preclinical research, including dedicated on-site veterinary surgeons, imaging specialists, and an expert team of staff. This infrastructure enables SAHMRI to support local and international biomedical and pharmaceutical companies in bringing cutting-edge medical technologies like TorqView to market.
The device was also supported in its early development by Flinders University’s Medical Device Partnering Program (MDPP), and key components were 3D printed by Optofab at the Australian National Fabrication Facility (ANFF).
Helping to pave the way for TorqView’s success is yet another example of SAHMRI’s commitment to delivering translational research and providing tangible benefit to the community.
TorqView has already attracted private investment and regulatory approvals, reinforcing South Australia’s growing reputation as a MedTech innovation hub.
PIRL acknowledges the facilities and scientific and technical assistance of the National Imaging Facility (NIF), a National Collaborative Research Infrastructure Strategy (NCRIS) capability.