About the SAHMRI Registry Centre
The SAHMRI Registry Centre was established in 2018 to unite the registry science and operational expertise available among our research community, to ultimately strengthen the Institute’s existing role in the registry space and expand our research capacity and training in this area.
The Centre brings together a collaboration of registries, including those based within SAHMRI and member registries based externally. The collaboration includes some of Australia’s most significant national registries with a wealth of experience and knowledge.
In doing so, the Centre provides for increased quality, efficiency, and cost effectiveness, maximising the value of the contributions and advancements made by member Registries. It also works on enhancing the capacity of researchers, both within and external to SAHMRI, by driving improvements in the quality of care and health outcomes for all Australians.
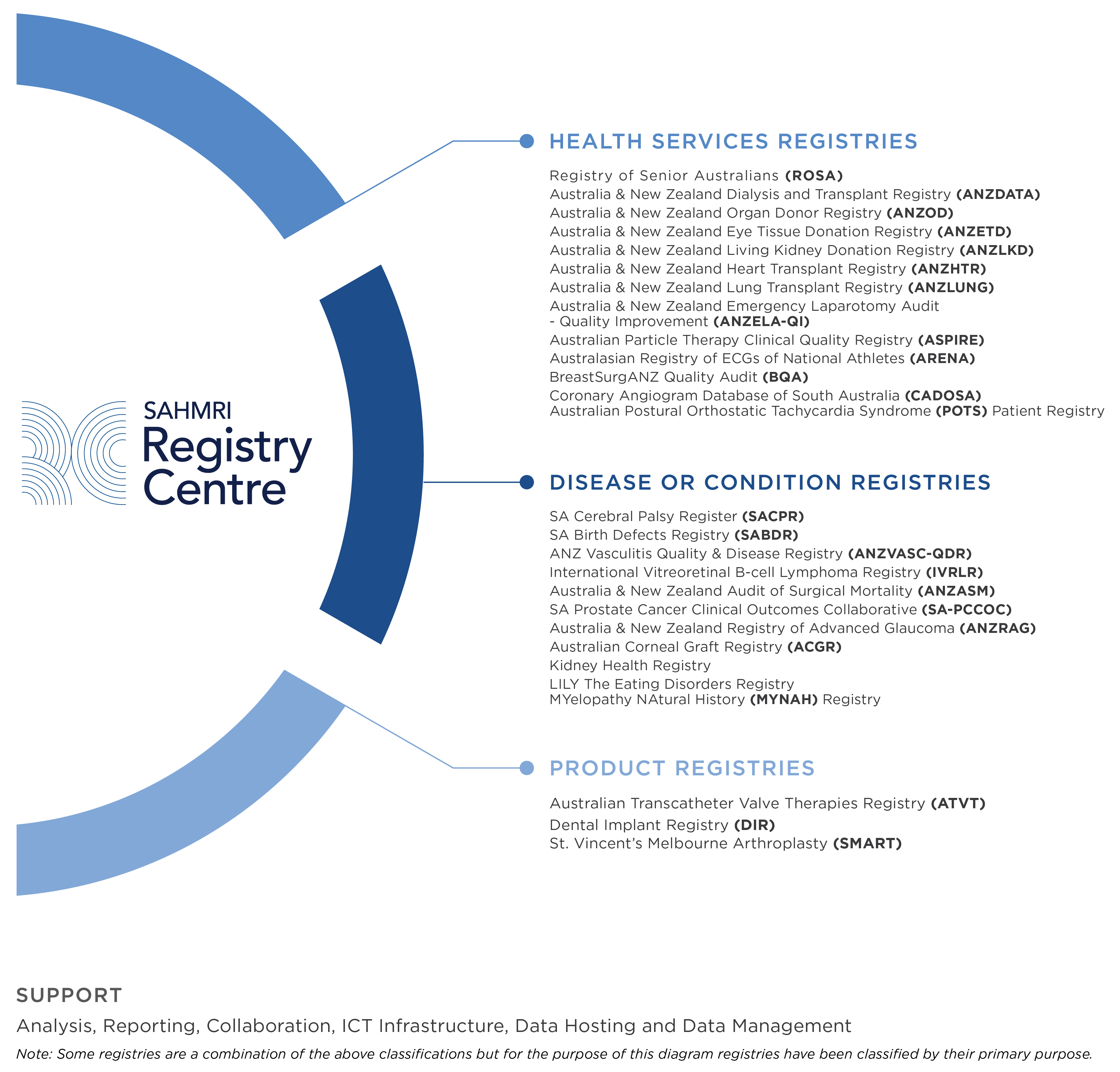
SAHMRI's Registry Centre provides an important and rich source of translational and observational research data. Well-designed registries, particularly at the population level, form the ‘third pillar’ of scientific research in conjunction with clinical trials and laboratory work.
The SAHMRI research community believes population-based studies founded by leveraging the information available in registries can represent the most reliable option for identifying the actual ‘real-world’ effects of interventions, treatments and outcomes in the general population.
The SAHMRI Registry Centre will provide a platform to enhance the enormous value of registry data, supporting the utilisation of other important data sources such trials, surveys, biobank data, administrative data sets, and information systems, through data linkage and research collaborations.
Read more about SAHMRI's Registry CentreThe Registry Centre supports the vision statement outlined by the Australian Government Department of Health in The National Clinical Quality Registry and Virtual Registry Strategy 2020-2030: "National clinical quality outcomes data are integrated into Australia’s health care information systems and systematically drive patient-centred improvements in the quality and value of health care to achieve better patient outcomes across the national health care system."
Registry Science
Registries are important databases that house uniform information related to specific diseases, conditions, demographics, interventions, or other unifying parameters for a specific cohort for a specific purpose. This information, which can include diagnoses, procedures, treatment pathways and outcomes, facilitates observational studies that can enable improvements in health care delivery and health policy.
Registry science is the required methodological, analytical, and other infrastructure required to operate, evaluate, and innovate using registries. A broad knowledge and skill set contributes to the operation of quality registries and facilitates their ability to impact health outcomes. These include structures such as good governance, ICT security and quality data management infrastructure, as well as complex project management expertise, a high level of clinical domain knowledge and academic research skills.
Governance
A collaborative funding arrangement between South Australian Health and Medical Research Institute (SAHMRI), Australia & New Zealand Dialysis & Transplant Registry (ANZDATA) and the Registry of Senior Australians (ROSA) supported the development of the SAHMRI Registry Centre throughout 2022-2024.
The Registry Centre is now moving to a model of project delivery through centralised project management using our expertise to expand and enhance best practices in registry science driving innovation and development across its member registries and the broader CQR sector where appropriate.
The SAHMRI Registry Centre Strategic Director works closely with the SAHMRI Executive Director, SAHMRI Registry Centre Advisory Group, and SAHMRI-based Registries on the activities of the Centre.
Meet the team
Purpose
The purpose of the SAHMRI Registry Centre Advisory Group is to advise and provide guidance on the work of the SAHMRI Registry Centre, specifically in the achievement of its key goals:
- Provide access to the best resources for building registry research, translational and technical capacity.
- Lead registry science initiatives through collaborative opportunities for our scientists.
- Develop strong national, international and industry collaborations.
- Continue to support a shared learning environment within our organisation.
- Become a leading national and international Registry Centre
Function
The Advisory Group is comprised of representatives of each member Registry, and performs the following functions:
- Guides the direction and strategy of the SAHMRI Registry Centre.
- Provides expert methodological and content area leadership for SAHMRI Registry Centre activities.
- Promotes registry science and registry best practice within the Registry Centre, nationally, and internationally.
- Strengthens the profile of the SAHMRI Registry Centre nationally and internationally.
- Provides guidance on activity-related priorities and consensus on the selection of activities for the SAHMRI Registry Centre.
- Contributes to the establishment of networks and fosters collaborations with other Registry Centres nationally and internationally.
- Generates and assists with dissemination of reports associated with SAHMRI Registry activities.
- Contributes to other meetings, forums and reports produced external to the Registry Centre.
The Centre provides support and expert advice across several domains:
- Registry Structure and Governance
- ICT platform and software development
- Data Management support/services
- Analytical support/services
- Potential collaborations and partnerships
- Access to SAHMRI Facilities to support the work of the Registry Centre
- Development and establishment of new Registries
- Funded projects that support the development of member registries
- Collaborative grant funded activities that support the development of registries
- Fee for service work that extends beyond the capacity of the Centre to deliver the required work at no cost
- Advisory support for a broad range of registry requests and activities
Contact Us
For enquiries, please reach out to the SAHMRI Registry Centre at registrycentre@sahmri.com.








