At PIRL, we pride ourselves on conducting rigorous, transparent, and reproducible preclinical research.
SAHMRI (including PIRL) has implemented a documented Quality Management System that is compliant with AS/ZS ISO 9001 (2015), thereby ensuring operational and management efficiency and effectiveness.
As a NATA recognised facility, our research can be conducted to OECD Principles of Good Laboratory Practice (GLP).
The PIRL site has both indoor and outdoor Quarantine Approved Arrangements (AA). An AA is a facility approved by the Department of Agriculture, Fisheries and Forestry for the purpose of receiving, storing and dealing with materials that are subject to quarantine. Our research support team can offer advice for import requirements and applications.
Quarantine AA and OGTR accredited facilities include:
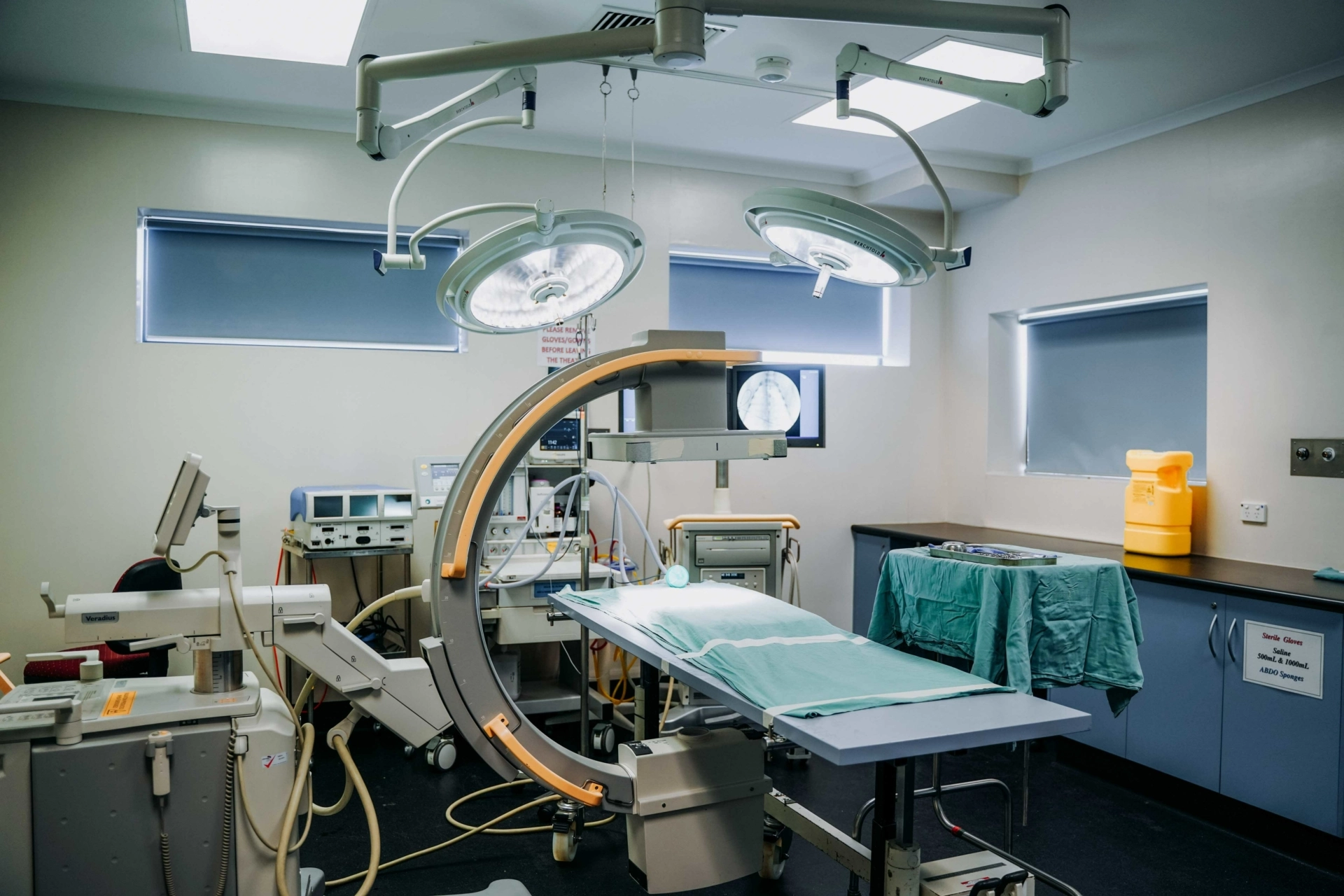
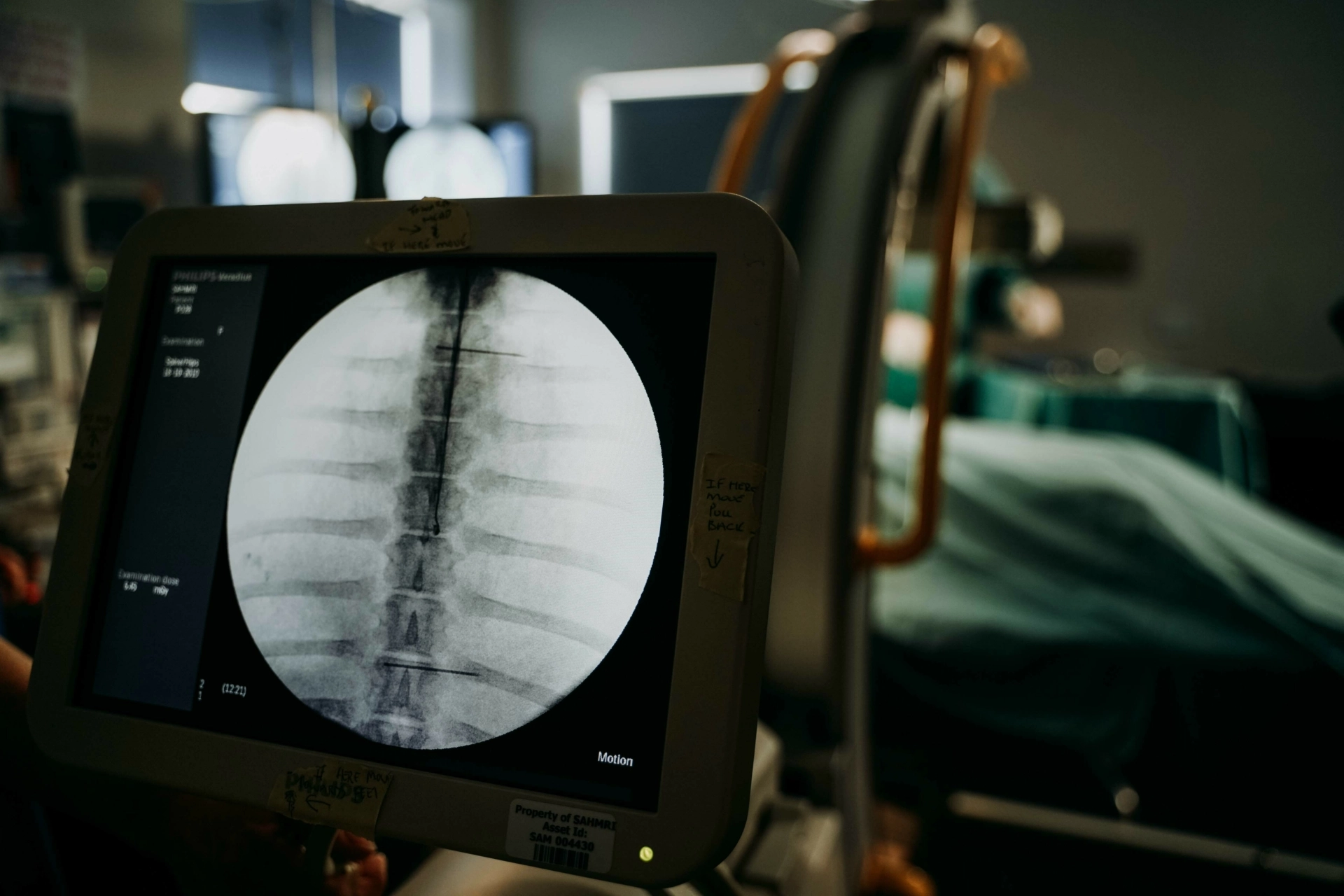
- BC1/PC2 Surgical theatres
- BC1/PC2 Indoor large animal holding
- BC1/PC2 large animal grazing facility (paddock)
- BC1 laboratory space and storage
- BC2/PC2 Small animal holding
- BC2/PC2 laboratory space including a biosafety cabinet