The level of support and service provided is tailored to each individual project.
We can provide support at all stages of the research process, including:
- Advice on experimental design and choice of animal models
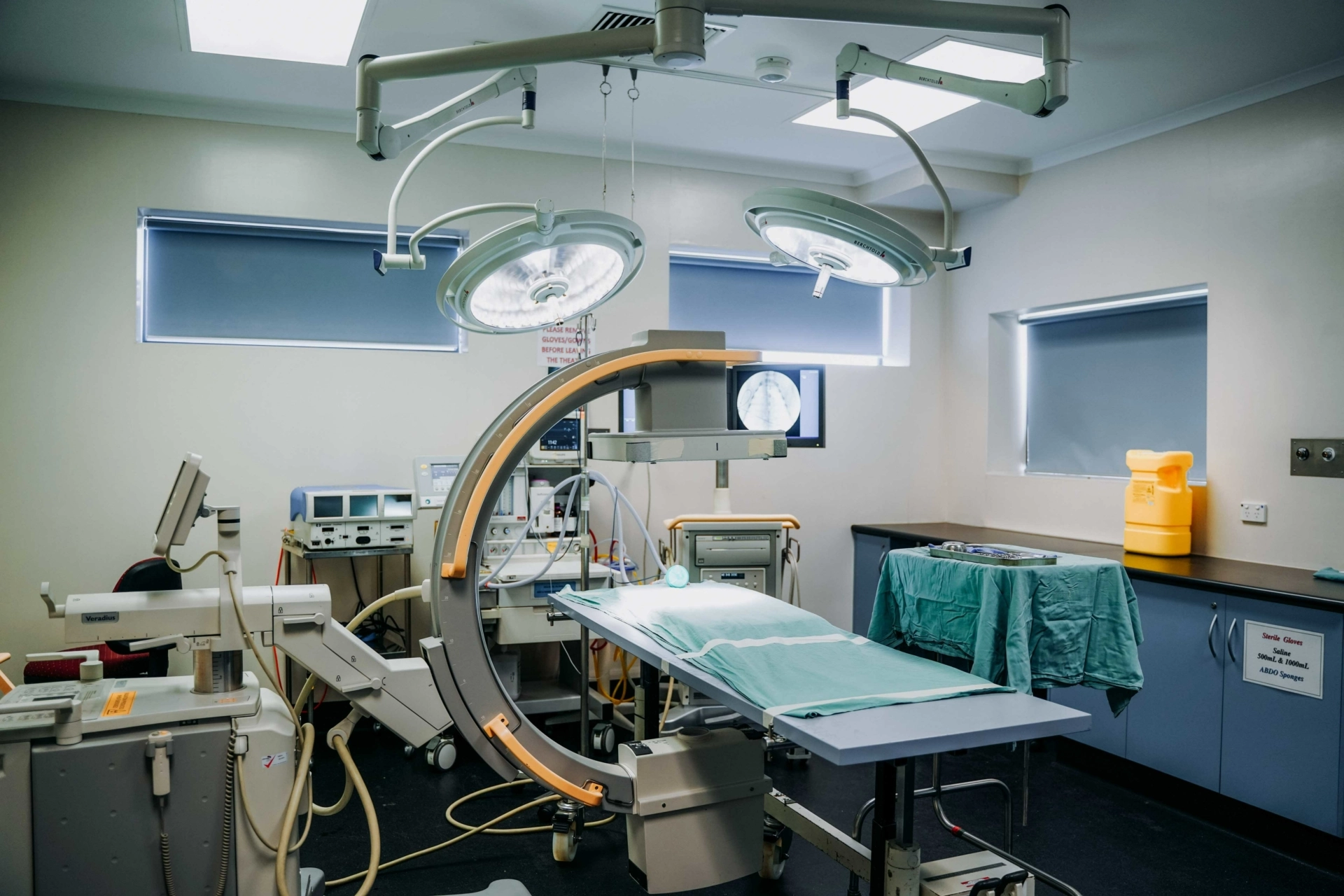
- Development of surgical techniques
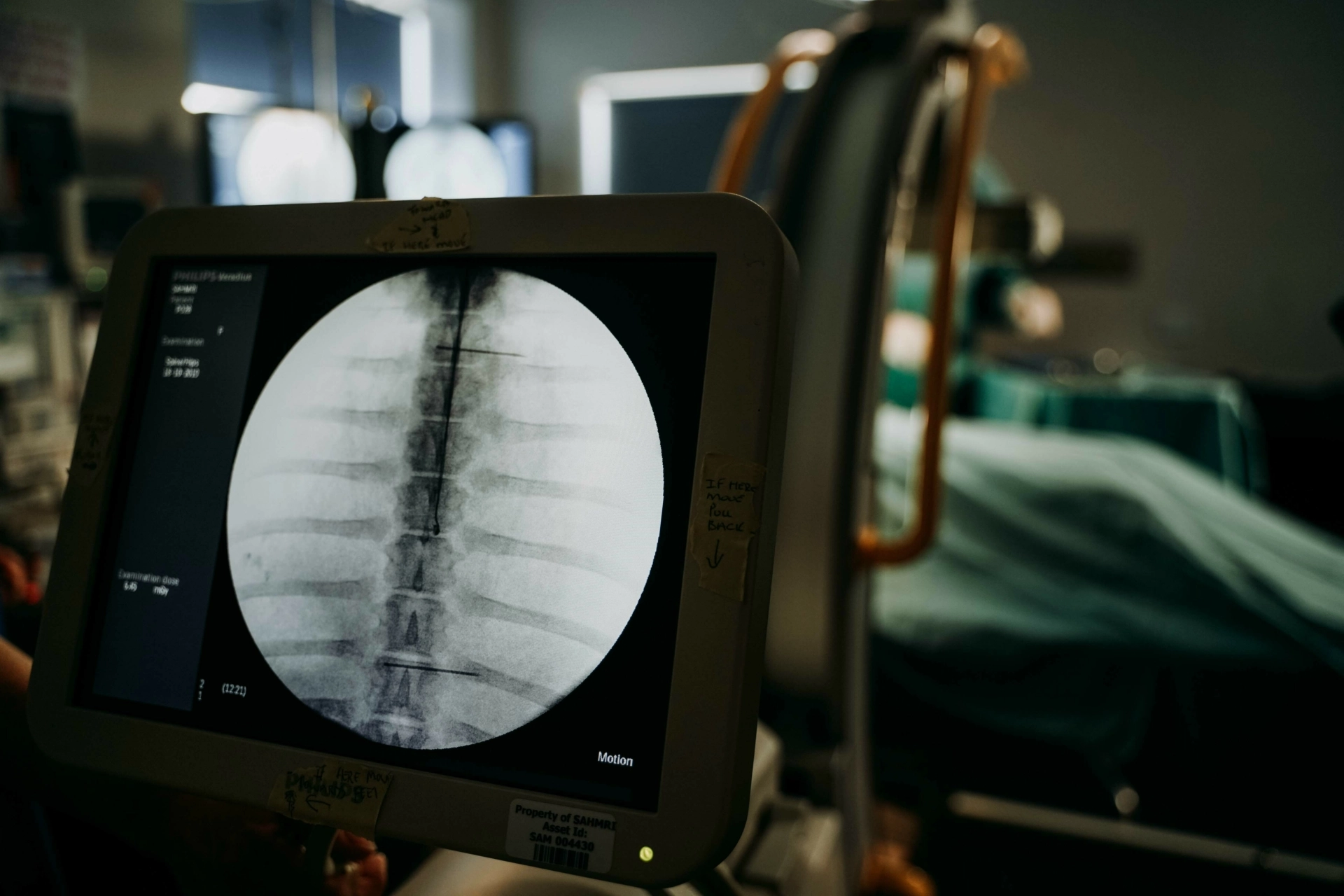
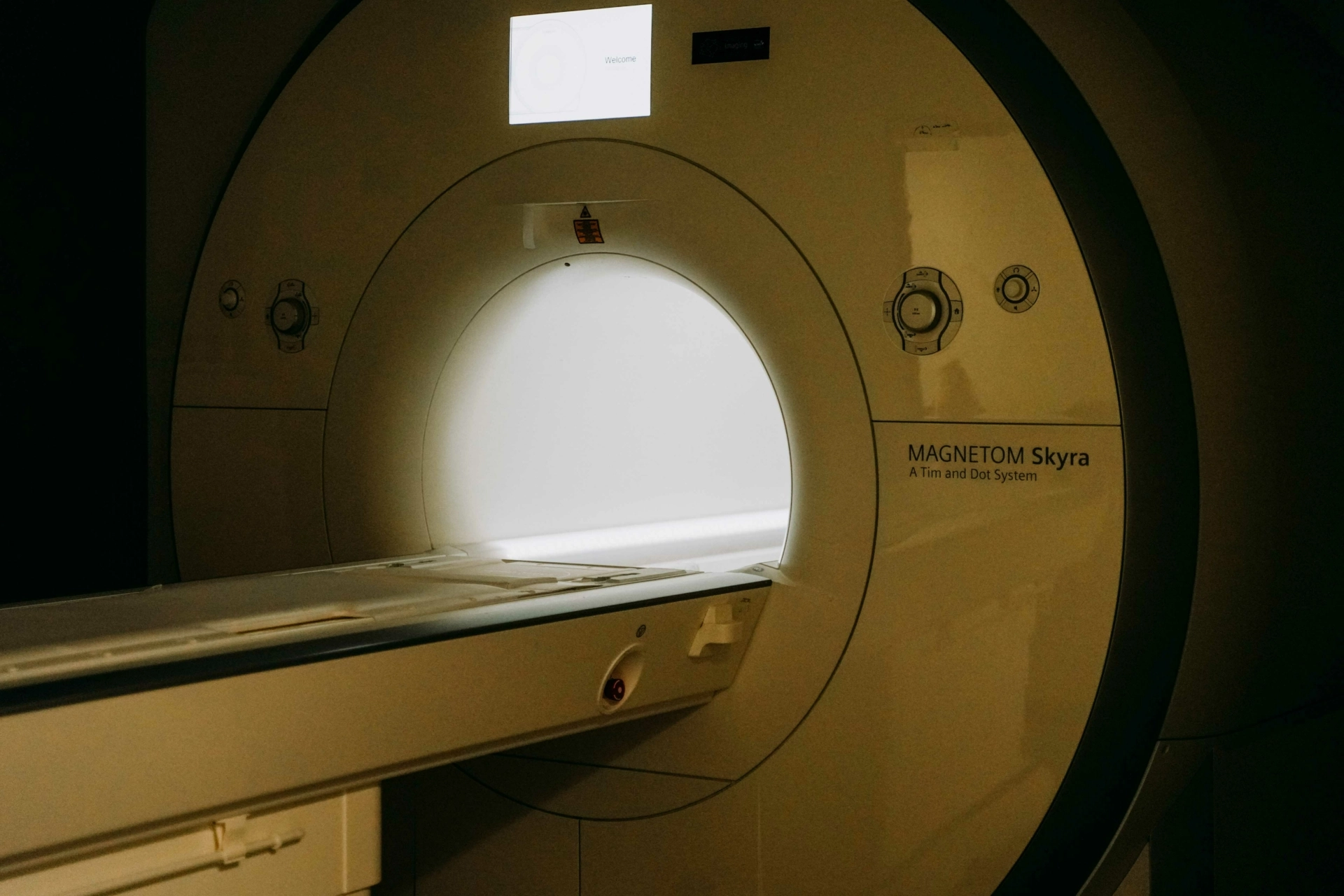
- Generation and processing of imaging data (e.g., PET-CT, MRI, fluoroscopy, X-ray, etc.)
- Preparation of applications to Animal Ethics Committees
- Approval from regulatory bodies including the Office of Gene Technology Regulatory (OGTR) and Department of Agriculture, Fisheries and Forestry (DAFF)
- Teaching, training, and workshops
PIRL offers a range of services for biotechnology and pharmaceutical companies wishing to establish safety or efficacy of their investigational new drugs and devices, in preparation for regulatory submission. We can conduct toxicity studies in rodent and non-rodent species per guidelines issued by regulatory agencies and international standards. Our research can be conducted to OECD Principles of Good Laboratory Practice (GLP).
Services

- Safety and efficacy of medical devices, including implants, prosthetics, and surgical instruments in a range of small and large animal models.
- Early Toxicology Screening - Dose Range Finding (DRFs) / Maximum tolerated dose (MTD)
- Pharmacokinetic Studies
- Acute Toxicity Studies - Single-dose Studies
- Repeat Dose Toxicity Studies
- Sub-Acute Toxicity - Repeat dose 7/14/28 Day Studies
- Sub Chronic Toxicity - Repeat Dose 90 Day Studies
- Chronic Toxicity - Repeat dose 120/180 Day Studies
Depending on the substance and regulatory requirements, additional studies may be conducted to assess specific endpoints such as neurotoxicity and carcinogenicity.