The Australian Particle Therapy Clinical Quality Registry (ASPIRE) is a prospective, observational, longitudinal study of paediatric, adolescent, young adult and rare adult tumour patients from a select group of tumour streams treated with radiation therapy.
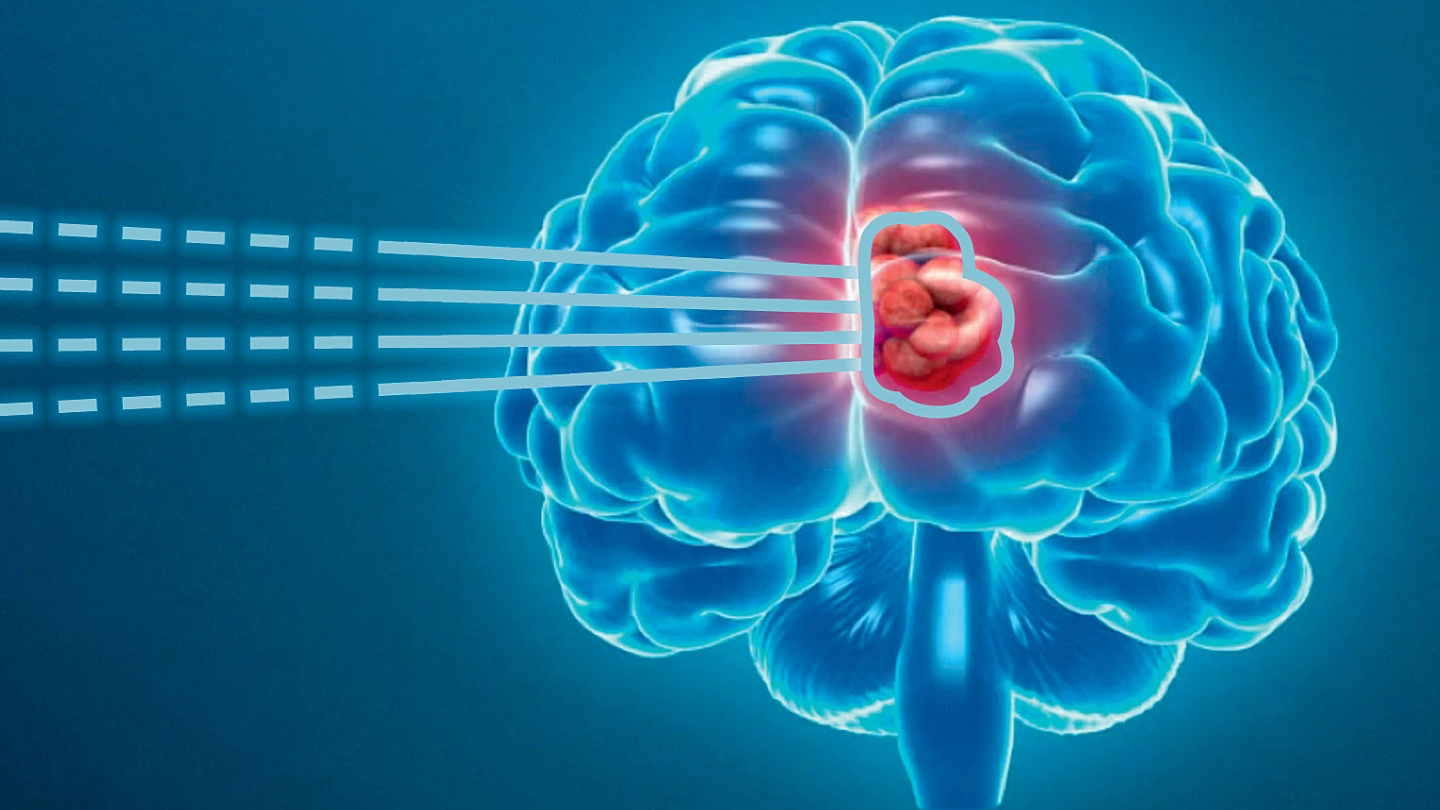
ASPIRE compares the long-term toxicities of photon and proton radiation therapy.

The registry enrols patients who have been treated with radiation therapy in order to better understand and compare the short and long-term benefits of the different types of radiotherapy. It collects patient information such as age, gender and post code, as well as clinical data including diagnosis, treatments and side effects.
There are no clinical interventions required for participants enrolled onto the registry. All enrolled participants will receive treatment, and follow-up care as pre-determined by their treating clinician, in accordance with the standard of care at the treating institution. All treatment interventions that participants receive, and all outcomes will be recorded on the registry database from the patient medical record.
It is hoped that the development of this registry will assist in describing the long-term effects and disease control outcomes for patients having radiotherapy as part of their cancer treatment.
Vision
The ASPIRE registry will help ensure equity of access for those eligible to receive treatment at the Australian Bragg Centre for Proton Therapy and Research (ABCPTR) which is under construction in Adelaide, adjacent to SAHMRI's headquarters. It will provide access to evidence-based research practices, resulting in the highest quality clinical care offered to patients.
ABCPTR is dedicated to becoming a centre of national excellence for paediatric, adolescent and adult health outcomes for patients with a cancer diagnosis that require radiation therapy. This will be achieved in partnership with national radiotherapy centres, key stakeholders and by leveraging the medical research capabilities of SAHMRI.
Support & Endorsement

The Trans-Tasman Radiation Oncology Group – TROG Cancer Research – is a proud collaborator with the Australian Bragg Centre for Proton Therapy and Research and has officially endorsed ASPIRE.
TROG Cancer Research is dedicated to improving the way radiation therapy is delivered to cancer patients with ongoing scientific research, clinical trials, and cutting-edge technology. Find out more here.

Grant funding to establish the ASPIRE registry has been graciously provided by the Hospital Research Foundation Group.
The funding provides for registry staffing and the necessary ICT infrastructure to support the registry until July 2024.
Visit here for more information on the impact the Hospital Research Foundation Group has in medical research and patient care across all South Australian public hospitals, universities and medical research centres.
More information about ASPIRE
SAHMRI – the parent company of the Australian Bragg Centre for Proton Therapy and Research (ABCPTR) – has successfully applied for certain cancer tumour types treated with proton beam therapy (PBT) to be listed on the Medicare Benefits Schedule (MBS).
As part of this application process the Medical Services Advisory Committee (MSAC) noted the uncertainty in the cost-utility modelling associated with PBT and recommended that a national registry be established to provide increased certainty around the claim of superior safety of PBT relative to conventional photon radiotherapy (PRT). ASPIRE was established in response to that requirement.
The application details and public summary document can be found here.
The ASPIRE protocol and participant information sheet received ethical approval from the Women’s and Children’s Health Network (WCHN) Human Research Ethics Committee (HREC) under the National Mutual Agreement (NMA) scheme on February 2nd, 2022 – 2021/HRE00394. This was followed by a site-specific agreement which was approved for participant recruitment to commence in the Department of Radiation Oncology at the Royal Adelaide Hospital in March 2022.
The registry commenced its national roll out in April 2022, with approval sought from the Northern Territory Government to commence ethics approval for participant recruitment at the Alan Walker Cancer Care Centre (AWCCC) at Royal Darwin Hospital. A Site-Specific Agreement (SSA) is in the final stages of approval for this site.