A cutting-edge clinical trial for people with a rare blood cancer is being expanded across Australia and potentially beyond.
The study is currently underway at two sites – the Royal Adelaide Hospital and the Austin Hospital in Melbourne – with additional sites to be established in New South Wales (2), Queensland, Victoria, Tasmania and possibly New Zealand.
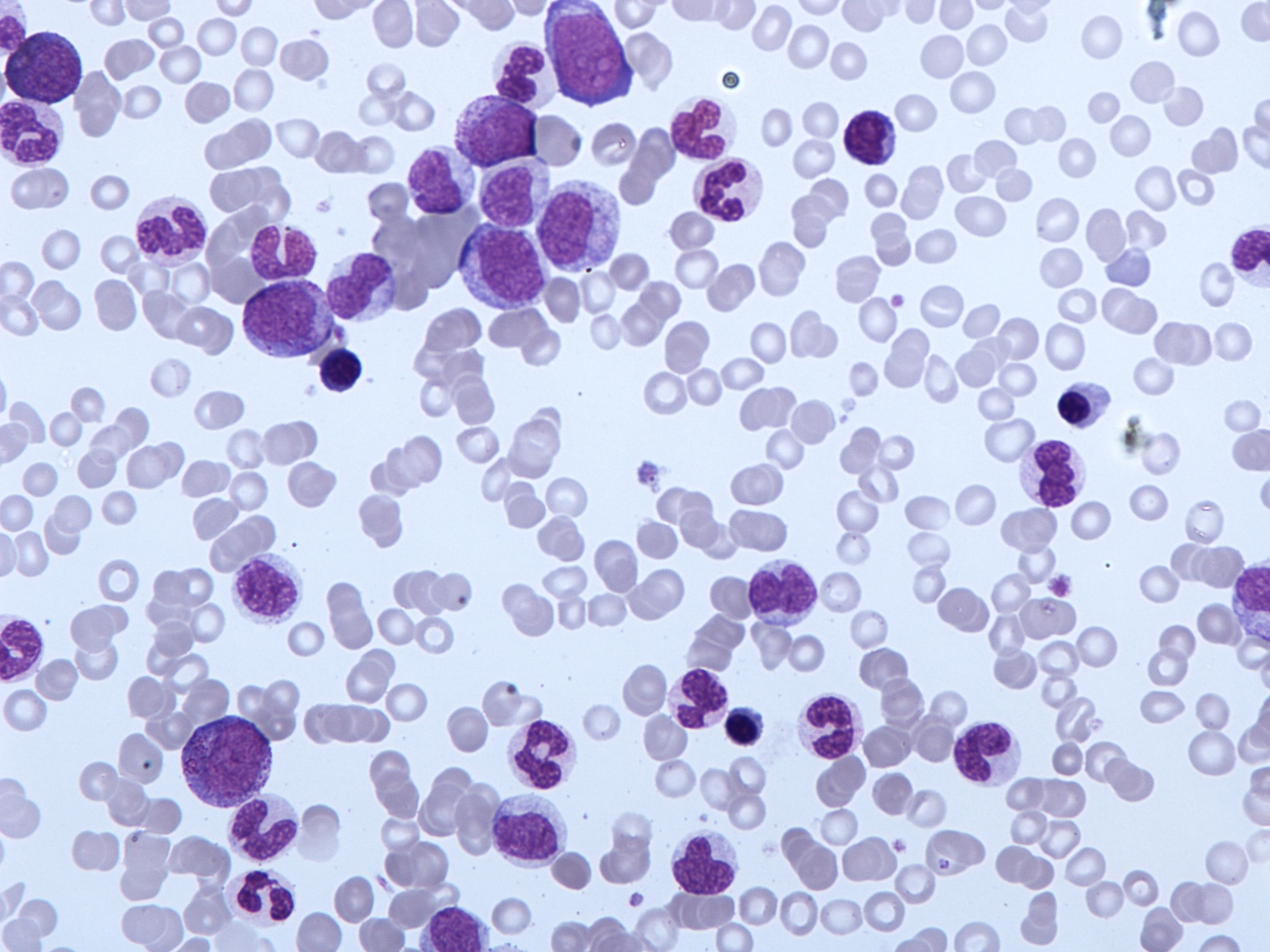
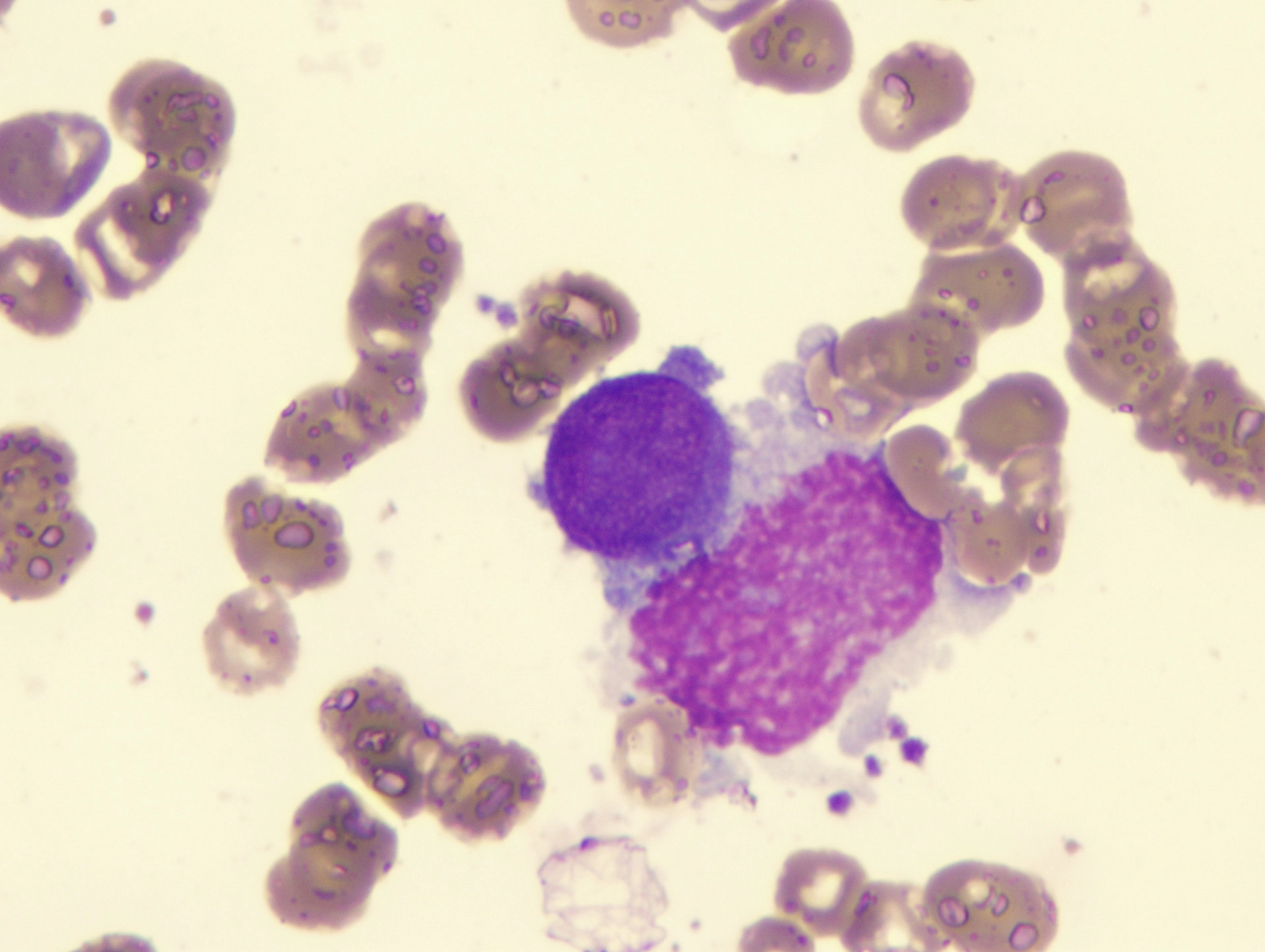
Associate Professor Dan Thomas, the head of the Blood Cancer Program with SAHMRI and the University of Adelaide, says more effective treatments for chronic myelomonocytic leukemia (CMML) are desperately needed.
“The prognosis for people with CMML is poor, with an average survival of around 30 months,” he said.
“Those survival rates have remained essentially unchanged over the past three decades, but in recent years, with the development of lenzilumab, we have cause to be optimistic about better patient outcomes.
"Although clinical trials have been slowed due to COVID staff shortages, doctors are really keen to support the study, including hospitals in New Zealand because there are virtually no treatment alternatives for this disease."
Lenzilumab has been developed by the US-based biopharmaceutical company Humanigen, which is supplying the treatment for this study. The trial is being sponsored by SAHMRI and supported by a grant from the Medical Research Future Fund.
Humanigen Chairman and CEO Cameron Durrant says expanding the number of study sites could enable more people who are diagnosed with this rare cancer to participate.
“Lenzilumab was discovered in Australia, so it is only fitting that we leverage the wealth of local knowledge that exists for lenzilumab as we continue clinical development,” he said.
“Associate Professor Thomas is a world expert in CMML and SAHMRI is a leader in precision medicine that assists patients in finding the proper treatment for their cancer.”
The Precision Approach to Chronic Myelomonocytic Leukemia (PREACH-M) trial is open to newly diagnosed CMML patients who haven’t had any treatment yet but are beginning to have symptoms including low blood counts, high fevers, enlarged spleens or high white cell counts and who generally feel unwell. Until now, these patients have been limited to having blood transfusions and supportive care.
Anyone who is interested in learning more about the study, or additional information can visit the Australian New Zealand Clinical Trials Registry. Investigators expect to see preliminary results from the study in the first half of 2023.
The incidence of CMML in the US, UK, and Australia is about 1,700 patients annually. As it is a rare disease, lenzilumab might qualify for certain regulatory and commercial advantages that could accelerate development and approval. Humanigen and the Principal Investigator are assessing regulatory pathways that may enable early results to support a regulatory submission and potential approval by the Therapeutic Goods Administration in Australia, which could be expanded through Project Orbis to the United States and the United Kingdom.