Immune checkpoint inhibitors (ICIs) represent a paradigm shift in cancer treatment, however, while these therapies are remarkably efficacious in some patients, response rates are widely variable between patients and tumour types.
Combination therapies with other agents such as immune agonistic antibodies, that target coactivating immune receptors, are now being pursued to enhance responses.
There are currently 25 different agonist antibodies being tested in 48 ongoing combination trials. Yet none have been approved to date, and only one has begun phase III trials. A major obstacle to their implementation is dose-limiting toxicity and, in some patients, serious side-effects including cytokine release syndrome and liver damage.

Excitingly, our preliminary data shows that the gut microbiota plays a critical role in mediating the toxicity of the agonistic antibody, anti-CD40. Germ-free or antibiotic-treated tumour-bearing mice had significantly reduced anti-CD40 induced immunotoxicity, cytokine storm, and severe liver damage. Critically, anti-CD40 anti-tumour efficacy was not impaired. Our exciting data suggest that microbiota targeted interventions could substantially reduce the immunotoxicity associated with anti-CD40 and possibly other agonistic antibodies, overcoming a critical roadblock in their clinical application. Unfortunately, antibiotic treatment is unlikely to a feasible clinical strategy as the efficacy of ICIs (which would be given in combination), is surprisingly dependent on signals from the microbiota.
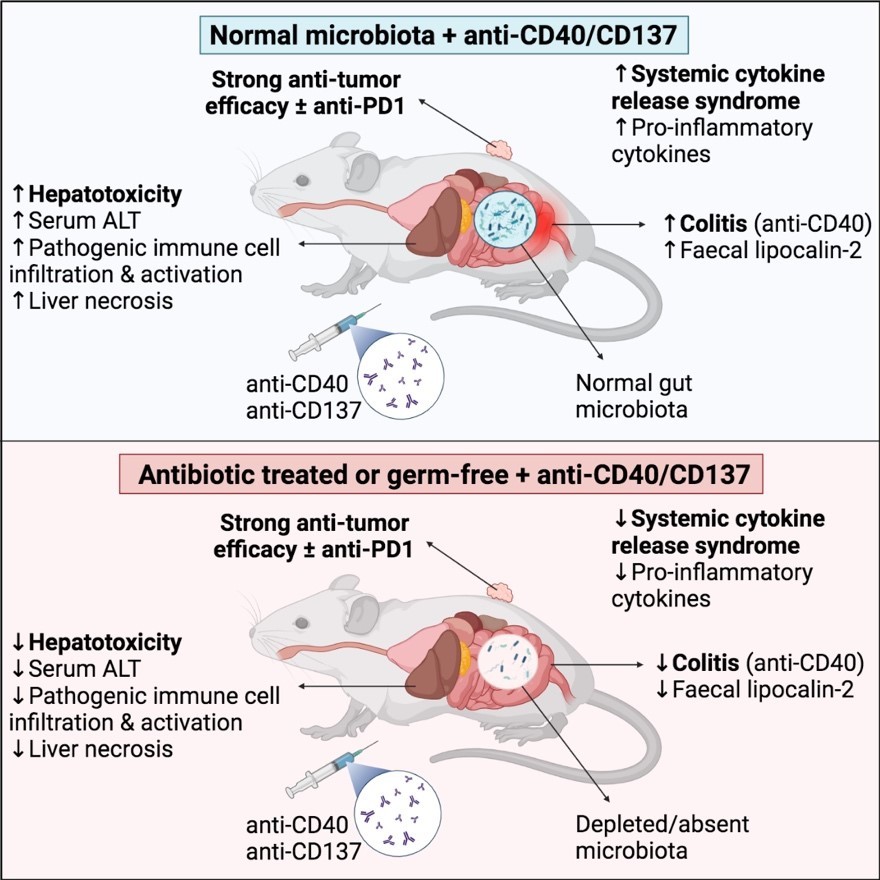
Image sourced from Cell Reports Medicine article available below.
Here, we will determine the role that specific bacteria, microbial metabolites, and host signalling pathways, play, disentangling the signals from the microbiota which promote immune agonist toxicity versus those that are required for the efficacy of ICIs. New strategies which reduce the dose-limiting toxicity of these drugs would simultaneously enhance their efficacy and safety, with significant clinical and health economic impact.
Resources
Project Leader
This project is funded by an NHMRC Ideas grant.
Funding also provided through Tour de Cure and Flinders Foundation Seed Funding.