Animal ethics refers to the moral principles and considerations that guide how we treat and interact with animals.
It involves evaluating the rights, well-being, and interests of animals, and making ethical decisions about how we use and interact with them in various contexts, including research and teaching-based activities.
SAHMRI prides itself on promoting best practices for animal welfare by following the governing principles of the Australian code for the care and use of animals for scientific purposes (8th Ed. 2013). The SAHMRI Animal Ethics Committee (AEC) adheres to the South Australian Animal Welfare Act 1985. The Act sets the legal requirements for the use of animals in research and teaching and regulates the institution’s Animal Ethics Committee function and reporting of animal-related activities.
SAHMRI’s AEC Operating Guidelines outline the institutional policies and procedures for the use of animals for teaching and research purposes. These guidelines extend to any personnel who utilise the SAHMRI AEC for the purpose of animal research or teaching.
All scientific research and teaching involving animals* requires AEC approval. All SAHMRI and partner researchers who wish to use animals for teaching, research, or experimentation within the SAHMRI Bioresources or PIRL facilities must obtain approval from the SAHMRI Animal Ethics Committee (AEC). AEC approval must be obtained before the use or involvement of animals in research projects or experiments, irrespective of the site involved, the ownership of the animal, or the source of funding.
If an institution or individual plans to use animals for research or teaching purposes, they must obtain a license. Please see “Do I need a License?” for more information. In addition, Researchers working with animals at SAHMRI are not permitted to begin any animal work until they have completed all relevant training associated with their project. Finally, any changes to an approved project are also subject to ethical review and must be approved prior to including them within the project. Please see “Submitting an Amendment”.
For any general inquiries regarding the Animal Ethics Committee, please contact secretary.aec@sahmri.com.
*Animal: any live non-human vertebrate.
The definition of an animal includes embryos, foetuses, and larval forms that have progressed beyond half the gestation or incubation period of the relevant species or have become capable of independent feeding.
Examples of animals as defined by the Australian Code:
• Vertebrates: (animals with backbones) e.g., monotremes, marsupials, placental mammals, domestic animals, companion animals (cats, dogs), laboratory animals, wildlife, pest animals, fish, rats, mice, guinea pigs, rabbits, non-human primates.
• Cephalopods: e.g., octopus, squid, cuttlefish, nautilus.
• Fish, including bony fish and cartilaginous fish (sharks, skates, and rays).
• Not insects, millipedes, annelids (worms), gastropods (slugs & snails), or spiders.
• Not shellfish (bivalves, mussels, oysters, scallops).
• Not humans
If an institution or individual plans to use animals for research or teaching purposes, they must obtain a license from the Minister in accordance with the Animal Welfare Act 1985 (SA). It's important to note that each state has its own act and specific requirements.
To apply for a license, the interested party must submit an application to the Department for Environment and Water (DEW) Animal Welfare Unit at least 28 days before the proposed start date. The cost of these licenses is $93 for a duration of 2 years. For more details and the application process, visit DEW.
Furthermore, individuals can contact DEW to verify the validity of their existing license or to check if their institution holds a valid license.
In cases where an entire project has received approval from an interstate committee, and this project includes work within SAHMRI, the ethics approval must be delegated from the interstate committee to SAHMRI.
Approval is obtained through a detailed written submission to the SAHMRI AEC outlining the project activities, justifying the use of animals requested, discussing the principle of the 3Rs (Replacement, Reduction, and Refinement), and recognising and accepting responsibility for the care and use of animals.
All animal ethics applications and amendments are required to be submitted via SAHMRI’s online database Tick@lab.
For guidelines on how to complete a new AEC application within this database, please read this guide.
Please allow ample time for submitting changes and requests to prevent potential delays. Ethical review is necessary for applications and modifications, and initial approval is not always guaranteed. The AEC may provide suggestions before granting final approval. To prevent any inconvenience, kindly submit applications and amendments well before the project's expected start date.
Additionally, all new applications must be submitted for pre-screening, and late submissions will NOT be accepted.
2026 Dates
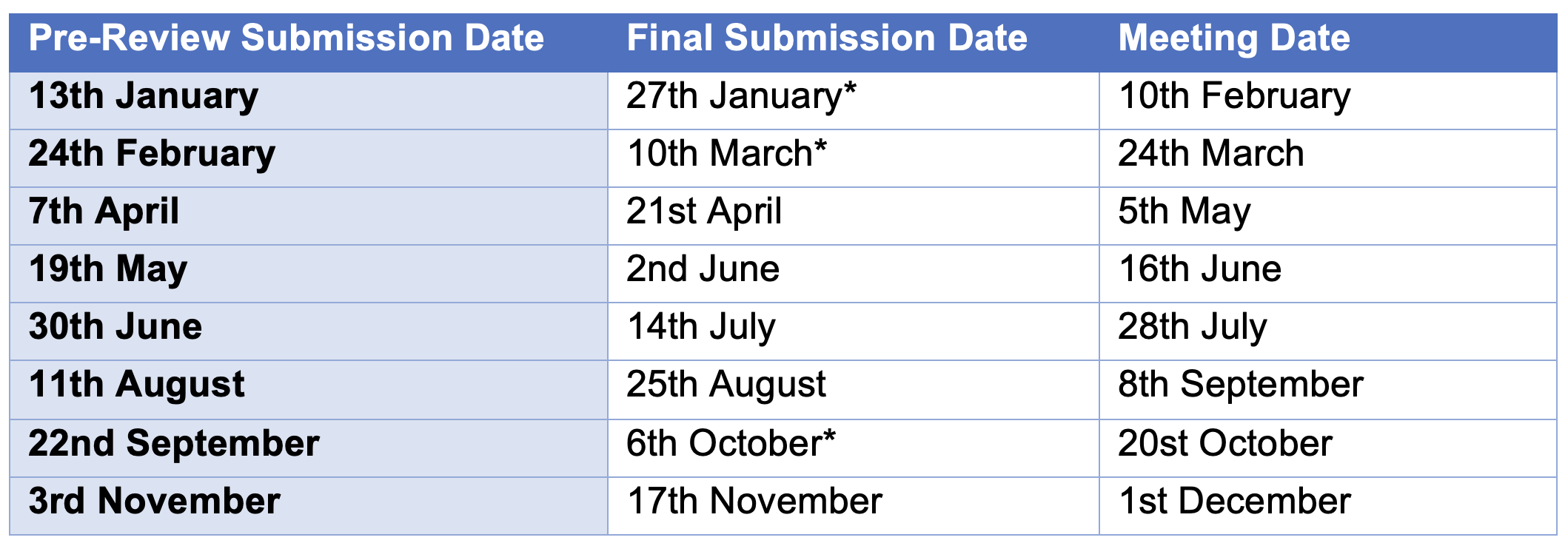
Items with an * occur near public holidays—please factor this into your planning.
If you have any difficulties with the database, please contact tickatlab_support@sahmri.com.
Researchers working with animals at SAHMRI are not permitted to begin any animal work until they have completed Animal Ethics Training and must repeat a training course every three years (or when the training has expired). People excluded from this requirement are those who have provided intellectual input to a project but who are not involved in the project and statisticians who will complete the post-processing of data. SAHMRI uses the ANZCCART ComPass Core (Phase 1) Online Animal Ethics Training Course.
As this course is external to SAHMRI please see their “Need Help” section located in the above ComPass link for technical difficulties. Please forward a PDF version of your certificate to the Animal Ethics Officer (AEO).
Any changes to existing approvals need to be submitted as an amendment to the Animal Ethics Committee (AEC). Amendments to a project must be approved by the AEC before the amendment is proceeded with.
The AEC emphasises the importance of submitting amendments at least 2-3 weeks in advance of ordering or utilising animals affected by the proposed changes.
All amendment applications need to be submitted online via Tick@lab. Please see the Create an Application Amendment guide.
All applications for amendments are considered at scheduled AEC meetings. However, the AEC may deem it appropriate for the Executive Committee (AEC Chair and Category C and D Members) to review out of session if the amendment is considered to be a minor change to the protocol.
A Minor amendment of a Protocol does not involve a change in the main aims of the project or the asking of a new scientific question. A Minor amendment would be a change that, in comparison to what has already been approved, has a minor or positive impact on:
- Animal Welfare
- The anticipated scientific or educational value
- The likelihood of meeting the project's objectives
Some examples of a minor amendment include:
- Extension of time of an existing approved project.
- Modification of procedures in previously approved projects.
- Change to Animals required (number, species, strain, etc)
- Minor increase in animal numbers.
- Change of Investigator or Other Personnel
- Where they are appropriately qualified or supervised.
- Additional tissues or samples collected post-mortem.
- Additional tests performed on samples approved to be collected
- Collection amount does not change.
- Additional of a new research location but using the same project methodology.
Amendments fitting the above classification of ‘Minor’ will be reviewed by the executive committee. The Executive Committee will aim to finalise this review within 5-10 business days, however, during busy times this may be longer, and the AEC Secretariat will communicate this with you directly. Any amendments outside of this scope are ‘Major’ amendments and require a full committee review. The AEC will aim to finalise their response following the major amendment review within 5-10 business days.
If the amendment is to be reviewed by the AEC, please submit it by the published deadlines.
2026 Dates

Scavenging is the collection of already available animal tissues and substances from discarded dead animals. These animals have been killed for other purposes, not for the collection of such tissues and substances. "Scavenging" tissue from carcasses is highly recommended by the AEC as it is an alternative to killing animals and greatly reduces the number of animals being used in research and teaching.
The opportunity for scavenging must not influence the decision to kill the animal, nor the time when this occurs, if this comprises animal welfare. Killing an animal specifically to collect tissues or substances for scientific or teaching purposes is also a scientific procedure, is not considered to be a case of “scavenging”, and therefore requires prior approval from the AEC.
Collection of organs, tissues, materials or substances from a living animal for scientific or teaching purposes is a scientific procedure and requires prior approval from the AEC.
Prior approval by the Animal Ethics Committee (AEC) is not a legislative requirement. However, the AEC should be informed when an investigator or teacher is "scavenging", this can be done via Tick@lab under “Scavenged Tissue” in an active protocol or by using SAHMRI's Scavenged Tissue Form and forwarding it to the Animal Ethics Officer.
Animal Transfers between approved projects can significantly reduce overall animal counts across various projects. These transfers are authorised solely by the AEC and approval is subject to AEC discretion, with a focus on preventing undue stress on the animals and maintaining ethical standards throughout the process.
The Animal Transfer Form is intended for transfers between SAHMRI experimental projects only and does not apply to breeding applications or transferring animals between different institutions.
Once completed, this form must be promptly sent to the AEC Secretary for submission to the next scheduled AEC meeting for official ratification.
Annual Progress Reports are required to be submitted to the AEC and are due on the date of approval of the project each year the project is active.
Final Reports are required to be submitted soon after project completion. Once the AEC has reviewed the Final Report, the application will be closed on the researcher’s behalf.
These forms will be available in your application on Tick@lab under Annual Review. The continuation of a project is subject to the fulfilment of annual progress reporting requirements for ongoing projects. Please refer to the application guide.
Alternatively, Annual Progress and Final Reports can be requested and submitted via email to the Animal Ethics Officer.
An Unexpected Adverse Event (UAE) is an unanticipated and undesirable incident or outcome that occurs while conducting a study or experiment involving animals. This event is not part of the expected or predicted results and may potentially harm the animal's health, welfare, or overall condition. These events could include unexpected health issues, reactions to treatments or procedures, injuries, or any other negative occurrences that were not foreseen. Proper documentation, analysis, and reporting of such events are essential to ensure animal welfare, ethical considerations, and the validity of the research findings.
- Act:
Immediately ensure the adverse impacts on the well-being of the animal are addressed. Alleviate any pain or undue distress to the animal by emergency treatment or humane killing. - Notify:
- The AWO Within the first 24 hours of the incident via phone or email
- The Manager of the facility in which the event occurred (Bioresources or PIRL)
- The Primary Investigator names on the application
- Report:
Please submit a formal report within 72 hours of the UAE via Tick@lab and notify the AEO and AWO when this has been done. Please refer to this User Guide for step-by-step instructions.
The report must include the following information:
- Ethics Number
- Animal ID, Strain, Age and Gender
- Location of event: Bioresources or PIRL
- Summary of events leading up to UAE
- Actions taken to alleviate pain or distress
- Attach Clinical Records Sheets, or other monitoring sheets.
- If applicable, post-mortem examination results
- Considerations for the prevention of future UAE and
interventions/corrective actions that may be taken.
When an animal dies unexpectedly, or is humanely killed due to unforeseen complications, a post-mortem should be performed by the AWO, Facility Veterinarian, or a person with appropriate experience or qualifications. Please place the body in the fridge to maintain anatomical structures if the post-mortem cannot be done immediately.
The AWO will investigate and advise of any interim arrangements required. This may involve modifications to procedures, urgent medical treatment, husbandry intervention, or, in exceptional cases, suspension of the project.
Please ensure all new protocols are submitted by the Pre-Review Submission Date.
2026 Deadlines and Scheduled Meetings

Items with an * occur near public holidays—please factor this into your planning.




