PLEASE NOTE: THIS STUDY IS NO LONGER RECRUITING PARTICIPANTS
We're seeking people with Molluscum Contagiosum (MC) to help us evaluate the safety and effectiveness of a study treatment.
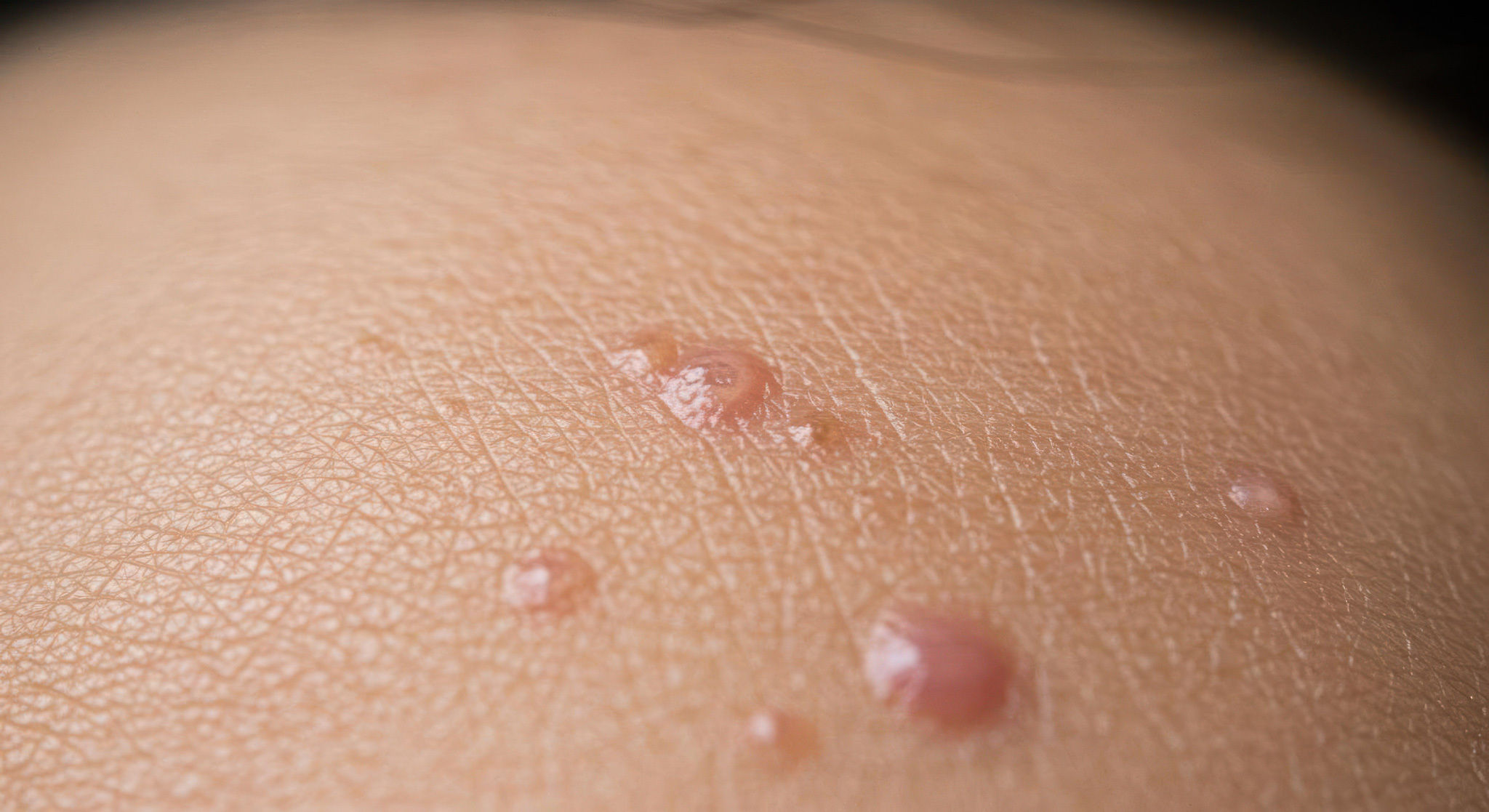
MC is a common childhood skin condition that causes raised, round bumps to form on the skin. It is highly contagious and currently has no treatment other than to wait for it go away on its own.
The condition is known to stay on the body for roughly six to eighteen months and there's no therapy or medicine available to treat MC in Australia. The only treatment offered involves burning, lasering or freezing the spots with liquid nitrogen.
This is why we're evaluating the effectiveness of a study treatment that can be applied directly to the skin called Zabalafin hydrogel.
Zabalafin hydrogel has been shown to have antiviral, anti-itching, pain-relieving, anti-inflammatory and antibacterial effects, which is why we hope this treatment could benefit people with MC.
Who can take part?
Your child may be eligible to participate if:
- They are between 6 months of age and 13 years old
- They have between 3 and 70 MC lesions
- Their MC has been present for less than 6 months
- You can bring them to SAHMRI for study visits on 8 separate occasions
Why should my child take part?
- Participants will be given a gel treatment that may treat or cure their MC
- You will be contributing to important research that may help people with MC in the future
- Eligible families will be reimbursed upon completion of the study
What does the study involve?
Participants will be randomly assigned (like flipping a coin) to receive either the hydrogel study treatment or a vehicle gel control (same texture and appearance but no active study ingredient).
There is a 66% chance of receiving the study treatment and 33% chance of receiving the vehicle control.
Both gels come in a tube and look the same, and you will not be told which treatment you are receiving.
Participation in the study will last approximately 4 months. After attending a screening visit, you'll be required to apply the treatment twice a day for roughly 4 months. There will be a study check-up visit every two weeks, with a maximum of 8 study visits to the clinic over the course of 4 months.

